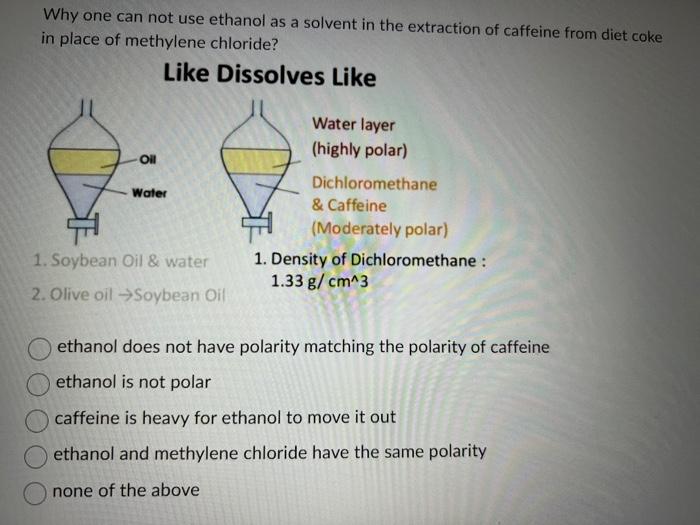
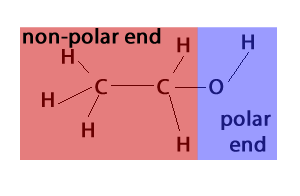
Is CH3CH2OH Polar or Nonpolar? - Polarity of Ethanol | Molecular shapes, Molecular geometry, Organic molecules

Arrange the following solvents in order of increasing polarity: a) ethanol b) ethyl acetate c) petroleum ether d) toluene e) acetone | Homework.Study.com

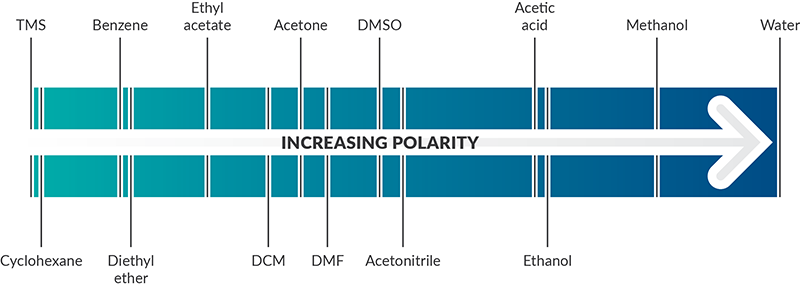
Pharmacognosy for all - Polarity index table of commonly used solvents To calculate the polarity index of a mixture of solvents : Polarity index of the mix.= (Polarity index of solvent 1 *


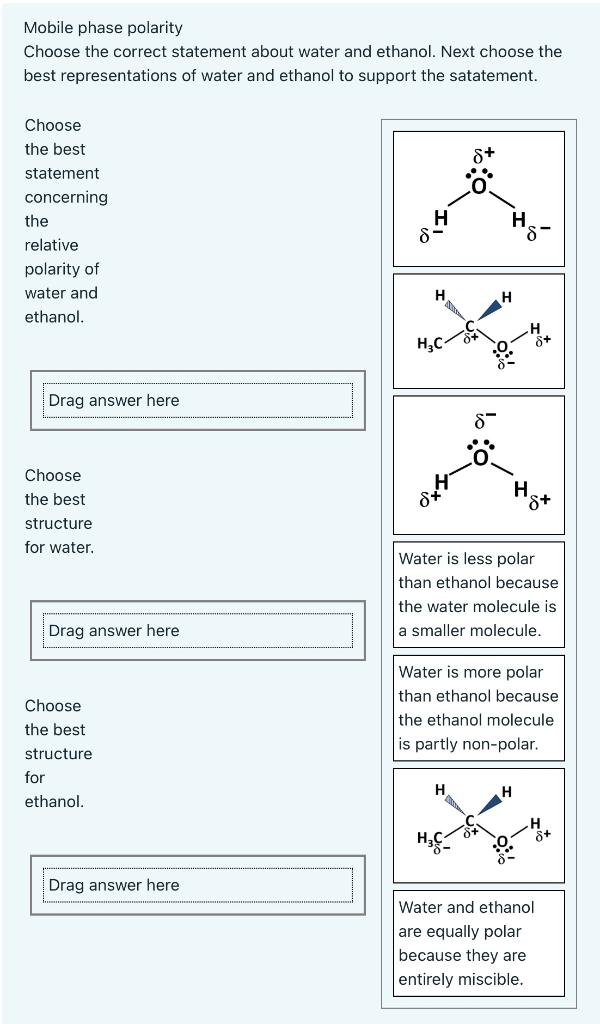





![Polarity values (literature data according to Ref. [18]). | Download Table Polarity values (literature data according to Ref. [18]). | Download Table](https://www.researchgate.net/publication/38014625/figure/tbl2/AS:668899632742421@1536489508874/Polarity-values-literature-data-according-to-Ref-18.png)